Meredith Leigh (2018). Assessing Soil Health using a Microscope Workshop
Meredith Leigh and Living Web Farms (2018). Assessing Soil Health Using a Microscope with Meredith Leigh. Video series. Retrieved from youtube.com/playlist?list=PLCeA6DzL9P4udTMBEM1Y9A3Ov3JNgyyYR (Parts 1–4) and youtube.com/watch?v=E7a6duF85ck (Part 5).
From description of the first video in this approx. 3 hour workshop: “The most essential question for farmers and gardeners to ask about their soil is, “Is it alive?” We will explore the diverse microorganism communities present in soil and why it is so important to keep them thriving and in balance. Meredith Leigh will explain the importance of a dynamic soil food web. View multiple different local participant samples and apply that information on your own farm or garden to build your own soil enriching strategies.”
(More slides and photos in the Highlights section)
Highlights
From Leigh and Living Web Farms (2018). Assessing Soil Health Using a Microscope with Meredith Leigh, Part 1. Video. Retrieved from youtube.com/watch?v=eG5eQroUSGo:
- 0:15–1:25: Brief introduction and reference to this workshop being based on the methodology of Elaine Ingham's Soil Food Web
- 2:23–3:08: “We're going to quickly be able to see the cumulative effects of soil management. I don't know how each one of you manage your soil, but based on what I tell you, when you look through that scope, you might be able to say, 'Mmmm, I till too much, or something like that'. So you'll have some really good visual cues that allow you to see how your management's affecting soil life. And then hopefully, you'll use your new knowledge to think about ways to change. I can say, I've been growing food on and off for 20 years — both as a commercial farmer, as a home gardener, working for non-profit — and I would say nothing has solidified the way I manage soil, and the management decisions I make as much as looking at the soil through the microscope.”
- 3:10–3:36: “I would say, as a veteran grower, you're constantly hearing different ways to manage soil or manage crops or use equipment, and you think 'oh, these are all just different options that I can use based on my preference or based on convenience', but taking a look at the soil has really changed my mind about some of that stuff, and I see certain management things as non-negotiable, now, in the way that I manage soil and the way I manage crop plans.”
- 5:34–5:54: “So hopefully, we're going to see some fungi. So these are really hard workers in the soil. They give the soil structure, ... depending on the type of fungus, if we're talking about mycorrhizal fungus ... they're actually acting as a carrier system for plants — seeking water, seeking nutrients and other things.”
- 6:25–6:29: On microarthropods, “they're kinda the choppers and shredders in the system.”
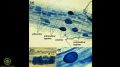
- 7:26–8:37: Overview of soil bacteria
- 8:02: A chart of the different types of bacterial morphology
- 8:10–8:19: “[The details of the different shapes] doesn't necessarily matter for us today, the main take-home is that you want to see a lot of different types of morphology in the bacteria.”
- 8:20: Examples of the morphology of pathogen-causing bacteria
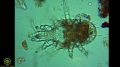
- 8:37–11:10: Overview of protozoa (incl. testate ones)
- 9:22: Examples of different types of protozoan cysts
- 10:01–10:16: “See these round blobs ... those are examples of cysts. And if they're super-ornate and double-walled, in general, they're amoebae, and if they're smaller and single-walled, they're going to be flagella or ciliates”.
- 10:20: On differentiating between flagella and ciliates
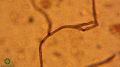
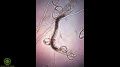
- 11:11–12:18: Overview of fungi
- 11:18–11:59: “What you're looking at here is a fungal hyphae or 'root', and they look like this... They generally have uniform diameter and they are branching, and if they're beneficial, you'll see little lines separating the cells — those are called the septa; if they have irregular septa, they're more likely to be oomycetes or not-so-good fungi. But the wider the diameter, the darker in colour and the more uniform the septa, the more beneficial the fungus. In general. So if you see something that looks tattered or frayed but it's a strand and you don't see septa and it doesn't have uniform cell walls, you're likely looking at a piece of organic matter or plant tissue and not looking at a fungus.”
- 12:18–12:52: On Oomycetes and Actonobacteria. “See those pencilled in(?) strands? [The oomycetes] still have uniform cell walls, for the most part, and you can see septa, but they're clear, very thin, there's a mass of them. The little balls that you see are their little chlamydospores — those are the ways that they're going to reproduce and grow. So if you saw this in a soil, you'd be like, yikes, this is not a good thing. This is indicative of a very highly anaerobic situation.”
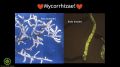
- 12:52–16:23: Overview of Mycorrhizae. “So, there's a bunch of different species, basically, but they're a particular type of mutualistic fungus that grows in association with plant roots.”
- 13:19–14:50: On plant exudates, and their interaction with mycorrhizae. “So if a little mycorrhizae spore sprouts, it has about 48 hours to sniff out the exudate, and make its way, with its hyphae, to that plant root. And depending on the type of mycorrhizae and the type of plant, it could colonise the outside of the root (an ecto-mycorrhizal association) — which is mostly on conifers” — or the inside (an endo-mycorrhizal association) — which is more common for what you'll see in agricultural crops — which is “where the fungus will grow inside, and in association with the plant root. So the plant tells the fungus what it wants, in terms of water, macro-nutrients, micro-nutrients, and the fungus sends out this extreme network of hyphae to go mining all that stuff from the soil and brings it back to the plant in exchange for continued plant exudates, or sugars, which is uses, basically, only to reinforce its cell wall.”
- 15:37–16:09: On the need for UV fluorescence or dyes to see the arbuscules (the site of nutrients exchange between the plant and the fungi). “You can, actually, dig [up] a root, wash it in a vinegar solution and then put it in an india ink — back and forth — until it takes up the india ink, and you can look at it under a scope, and sometimes you can see an arbuscule.”
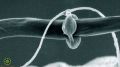
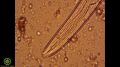
- 16:24–: Overview of nematodes
- 16:36–17:18: “For the most part, they're good guys. They eat different things: there are whole groups of them that eat bacteria, which is great — we need something to keep all that bacteria in check, then we have ones that eat fungi, and then we have ones that eat each other, and then we have some that spear our plant roots, and kill them. So, if you want to get rid of the ones that spear your plants' roots, you have to have the ones that eat other nematodes... So we need a good balance. In general, it's a higher taxonomic group, so in highly-disturbed soils, you're not going to find hardly any nematodes at all, and definitely not fungal feeders. I mean, think about it it — if there's no fungi in your soil, you're not going to have fungal-feeding nematodes.
- 17:28–18:58: On the morphology of the different types of nematodes. Also, “You'll almost only see a medial bulb and a terminal bulb on a bacterial feeder; if you see a nematode with fancy lips ... it's almost always a bacterial feeder. Fungal feeders have lesser defined bulbs, sometimes they won't have one, and then they have a very tiny, little spear [which] they inject into the fungal hyphae. Root feeders have a very strong spear, and it has a little knob at the end of it — it's pretty discernible if you're looking at a root feeder under the microscope, because they have to have a lot of strength, for shooting into the root, and killing it. Predatory nematodes have that weird 'tooth thing' right there near the mouth and all that skeletal-looking stuff, and then omnivores, they normally feed on fungus, but they'll feed on other things if there's no fungus there, and some of them will switch over and start eating roots, which is not awesome. So the point there is that if you have omnivores, they're not bad guys unless you're doing something wrong and you don't have fungus for them to eat.”
- 19:39–20:20: “There's also fungi that traps nematodes, which is really cool ... One, it creates these little lassos, and if a nematode puts even a part of their body near the lasso, it'll constrict and trap them, and it just grows into them and kills them, and it will kill any of these — it doesn't care — but it's one of the ways of keeping some of the other nematodes in check. ”
- 20:28–21:13: “Fungal-dominant soil is typical of permanent ecosystems like forests, but the more we can foster mature soils in agricultural situations, the more carbon we're sequestering, and the more nutrients are going to be available to plants. And so, one of the biggest impacts on me of this work is understanding that, even in alternative agriculture, there are things we've borrowed from convention that are not ideal. Continual soil disturbance keeps soil in an early successive state, where you will not find fungus communities, you will not find higher taxonomic groups, and you will find heavily bacterial situations.”
- 21:21–21:45: “Think about a roadside that's been blasted, what do early successive plants look like on that soil? They look like weeds and invasive groups, right? If you're constantly disturbing your soils, you're going to get early-successive organisms. Mostly bacteria, overgrowth of bacterial equals weeds, equals crusting of the soil, you know, all these things we consider unfavourable in organic agricultures.”
- 21:53: An example of a bacterial-feeding nematode
- 22:40–23:03: “You just try to get as close as possible to the mouth part — it's very hard to do, they're moving very fast, you have to change the focus, because even if you've smashed your droplet of soil sample under a coverslip, it's still 3um deep, and microscopic organisms are moving around within that depth, and so you're going to have to be focusing in and out through the depth.”
- 23:47–24:05: “Most of these organisms will cease to thrive at lower than 6ppm oxygen, so in general, as you move to anaerobic conditions, you're going to see [fewer] favourable organisms, and the answer is going to be aerate. That's, like, a really simple way to get the life back into the soil, first and foremost.”
- 24:06–: On the common non-taxonomic organisms
- 24:20–24:46: “Sometimes, if they're very geometrically exciting, they might be minerals from fertiliser that are floating around in the soil. If you're seeing a lot of that, it means you have a salt problem, and that you're feeding too many simple amendments, all the time, with all these loose minerals in them, and what you need to do is just chill out, let the life in the soil unlock everything, and send it out to the plants.”
- 24:51–25:04: Examples of aggregates and organic acids. Also, “So, humic acid is that colour — that's a great thing to see — if you're seeing aggregates in your soil it means you have decent aeration.”
- 25:04–25:46: “So, one of the things I didn't mention about the ciliates, in that group of protozoa, the ciliates — here and there, no problem, but if you see huge amounts of them, it means you're heading anaerobic. ... Other clues in a really anaerobic situation — if you see a bunch of creatures moving about really fast, I wonder if they're ciliates, and you're not sure because you can't see their little cilia or you're just starting out, well, do you also not see a bunch of aggregation? Do you also see a bunch of lactobacillii, which is an anaerobic bacteria which is really long and skinny? So all these context clues — the living and the non-living — come together to give you a picture and help you understand what's going on in your soil.”
- 25:54–26:24: “And I think that was one of the things that our compost was suffering from when I first started looking at it — we were over-working it, we were turning it far too much, and so we were not only just obliterating any fungal hyphae that were there, but we were also destroying structure and creating more of a, I mean it wasn't anaerobic but it was leaning that way, and it was just way overgrown with bacteria. And so it looked really good — it was crumbly, it was dark, it had all those visual cues you associate with good soil, but when push came to shove under the scope, there really wasn't much too it at all.”
- 27:01–27:43: “And then all the little guys like to live on and around those clumps, and so if you just want to look at the soil and see exciting things, zone in on your aggregates because you'll see, around the edges... I've seen ciliates scurrying all around an aggregate, vacuuming all the bacteria off the top of it, or I looked at some compost tea ... and I saw these little, baby bacterial nematodes ... going all around an aggregate, it was amazing, the depth that I was witnessing in this tiny, little soil sample.”
- 27:50–28:19: Examples of cellulose, seed coat and plant tissue
From Leigh and Living Web Farms (2018). Assessing Soil Health Using a Microscope with Meredith Leigh, Part 2. Video. Retrieved from youtube.com/watch?v=D_FGijGBZW4:
- 0:14–: On the basic lab supplies needed for this work (test tubes, microscope slides and coverslips, pipettes, chlorinated and non-chlorinated water)
- 2:27–4:16: In preparing samples for examination under the microscope, you want to fill a test tube with about 1mL of 'representative' soil (i.e. a mix made up from multiple samplings in the field, and banged lightly to help it settle down in the tube), and then bring it up to 5mL using non-chlorinated water to create an initial solution necessary to see the larger organisms, if they're there. If you're actually wanting to quantify the whole range of micro-organisms, you'll need to dilute this initial solution approx. 1000-fold in order to be able to estimate bacterial densities, etc.
- 4:16–4:38: On how to (gently) shake the mixture so as not to destroy the micro-structures and/or shock micro-organisms into their testate forms — roughly 1 up-and-down shake of the elbow per second, for 30 seconds.
- 4:54–5:33: “You know, a lot of the people I've worked with were used to a vacuum-process, where they were shaking it up and mixing it, and they were like, actually, oil-immersion microscopy, and they were vacuuming all the water out of the samples; they're killing everything anyway — these things, they're like you an me, if you smashed me and sucked all the water out of me, I would die, so these little critters are not much different. So the reason Elaine's methodology is what it is, is that we're looking at these things in real-time, we're looking at them, in the water, moving around, and that's why you don't have to have a super-powerful microscope, that's why they're generally affordable.”
- 5:53–9:15: On applying the soil sample-solution to the slide
- 9:15–13:45: Quick introduction to the microscope, its controls, and what they do
- 12:42–29:27: Example scan, including an explanation of parfocal lenses; how focusing and shadowing can help with the identification of the constituents present; how the presence of air bubbles under the coverslip can lead to 'false' (and uniform) motility; how clay particles can be mistaken for bacteria, due to their size (and Brownian motion); and how an 18x18mm slide has about 20×20 fields at 400x total magnification
- 23:17–23:44: “Elaine says that, if we're doing really well, ... there'll be a fungal hyphae in every field of view, and that we should have one nematode per slide.”
From Leigh and Living Web Farms (2018). Assessing Soil Health Using a Microscope with Meredith Leigh, Part 3. Video. Retrieved from youtube.com/watch?v=4oA0Qf_8nPk:
- 0:15–: Digression on the Johnson-Su Compost Bioreactor
- 2:20–2:37: “And then he removes the pipes after 24 hours, and the mycelium of the fungus — in 24 hours — is already so advanced, that it will hold these holes, that the pipes had created, even if you take the pipes out. It's awesome!”
- 2:50–3:52: “It's ready in as early as three months, but he leaves it as long as he can. ... Then it just ages, basically. But he said that he's seen micro-organisms in there that people haven't seen in 100+ years, which is really exciting. He did a presentation at the Soil Carbon [Trading Roadshow] in Australia, and if you're into this kind of thing, watch it, because not only does he go over the design again, but he also talks about what he's seen, and how he uses the compost. Because, depending on the material he uses to compost, he gets a different consistency of compost — some of the stuff he was showing was this really sticky, it was like a picture of his hand just, 'ribboning' the compost up, and that stuff he's using to coat seeds or pumping it through his irrigation, using it for extracts.”
- 4:32–4:50: “He gets really cold [ambient] temperatures, and really hot temperatures, and the compost is fine. The beauty of it is he uses any material he wants, and he never turns the pile — that's why he gets such rich life in it, because he's not disturbing it, and he's able to mature the system.”
- 5:48–: Preparation and examination of second soil sample
- 8:17–9:10: On the cell structure and use of wood chips in producing compost: “It's a substrate, for sure, for the organisms to live on; it's also a food source for, primarily, fungus, after it's been broken down by other organisms. But one of the most amazing facts that I learned recently is that, a fungus, if it has been present in the soil and has been able to build up a really strong body — so strong cell walls, strong hyphae — that's basically strictly carbon, the skeletal body of the hyphae, so even if that fungus dies, say that soil goes anaerobic, even briefly, and a bunch of anaerobic bacteria come in and suck all the cytoplasm out of that fungus and it dies, that carbon skeleton of the fungus will stay in the soil for 100–200 years. So it's still giving you the structural benefit and its still sequestering the carbon. So to have had fungus at some point is so much better than to never had fungus at all.
- 9:36–10:48: “Okay, so if I spray this grass with a full concentration of wood vinegar [the vinegar that comes from pyrolysing bio-char], what happens? Well the grass dies, but what happens to the soil? And I was able to see that the fungus was being attacked by anaerobic microbes, and that they were congregating all around the fungus. So I looked at the soil before spraying, I looked at the soil after spraying, and right after we sprayed, all the fungi in the soil was surrounded by bacteria, and the only thing I can assume is that those are anaerobes, and that they're sucking out the cytoplasm, releasing acidic glues. And so, great — you have an organic pesticide and it kills your grass. The question is, what's it doing to everything else, and the bigger question is what do you do after you spray? So, if I spray that grass, and all those bacteria go and suck that fungus dry, well, then say I go back and throw some really awesome aerobic medium on top of that, and I get going in the right direction again, then it's okay — I'm probably going to let the good guys win. But if I keep hitting it with the most organic, but super-acidic anaerobic products that I can, I'm certainly going to be killing the beneficial life in the soil, or keeping that early successive state.”
- 18:31–18:55: On the effectiveness of (ecto-)mycorrhizal supplements, “it just depends on the source of the mycorrhizal inoculant — if they've chopped up roots to produce it, it's not going to be as effective. But if they've actually isolated extramatrical spores through a scope, then that's going to be more effective, because you know that you have something that's going to 'sprout' and find a root.”
- 19:12–19:40: “So the way we measure [fungal hyphae] is we take the smallest bacteria in the field of view — and you consider that one micrometer — and you add up how many of those bacteria it would take to get across the width of the fungus, and you measure the length of the fungus based on the length of the field of view, and so great than, I believe, 2.5um wide is considered beneficial.”
- 22:21–22:26: “The more I look at soil under the microscope, the less I want to till ever again.”
- 22:35–23:43: “Say an extramatrical mycorrhizal spore does end up in your soil, or is there, it has 48 hours to find a plant root after it germinates — that's really not that much time. So even in highly organic systems, if you have constantly tilled soil, or at least annually-tilled soil, and complete annual crops that are all going to be ripped out — all those roots are going to be ripped out of the soil — and then yeah, maybe you're planting cover crops, but how much time are you actually giving mycorrhizal to establish, and to thrive? Because, say you have a really great endo-mycorrhizal colonisation of your onion crop, but you go in there and harvest all your onions, you disturb all the hyphae. And if there's no perennial there to support that network, it's just going to die, and you're going to have to re-establish it. So this is a huge argument for inter-cropping perennial and annual species, this is the argument for silver-pasture(?), this is the argument for permaculture, no-till, all that stuff.”
From Leigh and Living Web Farms (2018). Assessing Soil Health Using a Microscope with Meredith Leigh, Part 4. Video. Retrieved from youtube.com/watch?v=vc-RKCxf17k:
In this video, the participants of the workshop get to prepare and examine their own soil samples and solutions. Questions and answers, and comments are made throughout.
- 24:15–24:42: “I think it's plant tissue. See how it looks crumpled or 'smashed'? Fungus will never look tattered or torn like that. It'll either look broken, like a clean break, and it'll be really rigid because of it's very hard carbon structure.”
- 46:24–47:40: “The other thing I've done is, if anybody makes Sauerkraut, it's really fun to take the brine of your Kraut and look at it under the scope. You'll see anaerobic conditions in there, under the surface, a lot of lactobacillus. Or beer, wine that's fermenting, if you want to look at the yeasts, which are anaerobic fungi. ... But if you end up getting a scope and you're, like, 'how am I going familiarise myself with all of the morphology?', find things that you know will have yeast in them, or lactobacillus, and look at them, and you'll learn that way. Looking at anaerobic compost teas or swampy soils or soil that has been left in a closed, air-tight container — see what you see. And you learn more from finding bad things, than looking at good things over and over and over again.”
- 47:53–49:11: Example of a poster / wall reference showing the major groups of microorganisms. As of March 2022, this poster was not available, either in print or digital form, however, the other Microbes Identification Chart shown below is a decent alternative. (Follow the link to get the high-resolution PDF version rather than the lower-resolution preview image.)
From Leigh and Living Web Farms (2018). Assessing Soil Health Using a Microscope with Meredith Leigh, Part 5. Video. Retrieved from youtube.com/watch?v=E7a6duF85ck:
In this video, Meredith goes through a slide show, pointing out how different micro-organisms appear under the microscope.
- 20:41–21:20: “I always have, in my non-growing spaces, I'm putting [down] pretty unrefined wood chips or that leaf mould ... would be an awesome thing to be mulching a pathway with, especially if it's not highly refined because, even though you're going to be walking on it, if the particles are big, you're not going to be compacting it so much that it goes anaerobic. But it's going to be a really stable carbon source, and a really rich food source for nematodes, for fungi, and for stuff like that, and it's going to be there, even while things are changing in the growing spaces.”
- 21:55–22:32: “You can also look at the life in the soil. Sometimes, if you see a few ciliates, a few lactobacillii, it could be that it's not going anaerobic but that it's actually coming out of being anaerobic, so look at your other context clues and do time-core samples. One of the things about lactobacillus — those long, skinny, squarish rod-bacteria — is that they will fight off other anaerobic bacteria, so they're not the worst kind of anaerobic bacteria to have in your soil, they're just a cue. But if you're seeing them and actinomyces and ciliates and low aggregation, you might think, 'Hmm, I don't have very good aeration'.
- 22:36–23:00: “If I'm putting fungus in the soil — even if I'm renting my house and I know I'm moving out in a year — if you can tend that soil and fill it full of fungus, then you're doing everybody a favour. Even if the next person comes along and kill it, you're still leaving carbon in the soil, so that's really powerful for me.”
- 31:49–32:18: “And then there are people who say, if a compost has sat for a certain amount of time, then you need to feed it. So you would give it oat bran, oat meal or things that the fungi want. Elaine says no molasses — it's too much of a simple sugar, it's a bacterial food. So fish hydrolysate, oat bran, still-cut(?) oats.”